Lecanemab selectively targets the amyloid protein thought to be the most damaging to brain cells
In 2023, the Food and Drug Administration (FDA) approved lecanemab (brand name Leqembi) for the treatment of Alzheimer’s disease. Lecanemab is the first widely used medication in a new class of anti-amyloid agents for the treatment of patients with mild cognitive impairment (MCI) or early stages of Alzheimer’s disease. This is the first new Alzheimer’s drug with full approval in 20 years. This is also one of the first drugs to potentially slow the progression of Alzheimer’s disease.
“Alzheimer’s can be devastating, not just to patients, but to their families and communities as well,” said Gregory E. Cooper, M.D., Ph.D., chief of adult neurology and director of the Norton Neuroscience Institute Memory Center. “This therapy gives hope to those who have been affected by Alzheimer’s.”
Mechanism of action
Lecanemab selectively targets the amyloid protein thought to be the most damaging to brain cells. Research has shown the drug reduced brain amyloid levels and slowed the rate of cognitive and functional decline by 27% over 18 months, compared with placebo. This drug is most effective for patients with early-stage dementia.
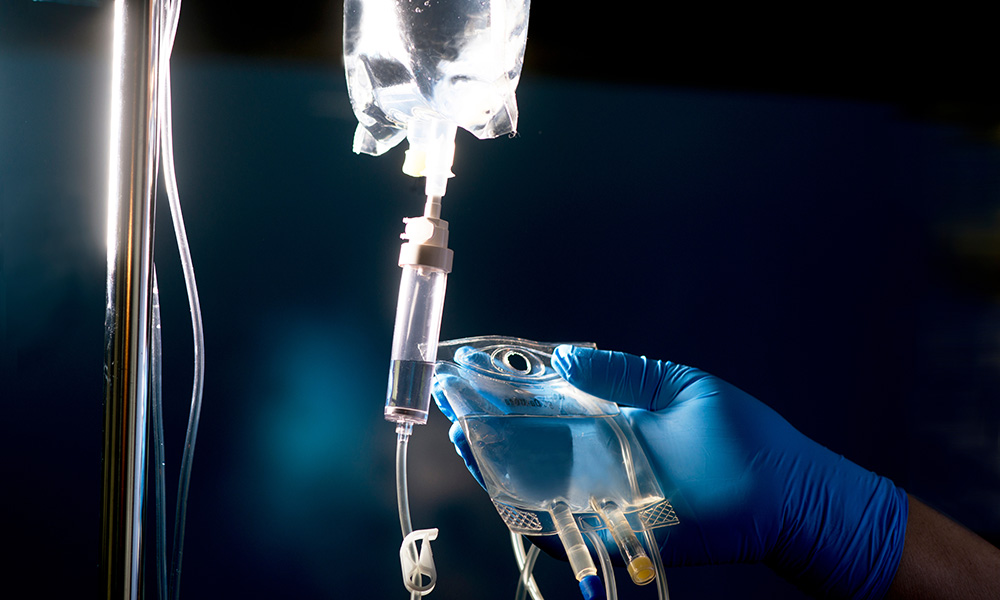
Delivery
Lecanemab is usually administered as an intravenous infusion every two weeks in an infusion center. The infusion is administered in less than an hour, with extended careful monitoring afterward. Repeat MRI scans can be completed three to four times during the first year to monitor for any side effects.
A subcutaneous injection is anticipated, possibly as early as 2025.
Side effects and risks
Studies with lecanemab show significantly lower rates of some side effects than do published trials of other, similar drugs such as aducanumab, thus giving the drug a preferred safety profile.
- Infusion-related reaction, including transient symptoms of chills, fever, rash, body aches and flushing occur in about 1 in 4 patients. Most side effects (96%) were reported as mild to moderate, with 75% occurring after the first dose.
- Amyloid-related imaging abnormalities (ARIA) with edema on the brain occurred in about 12.6% of trial participants and 1.75 in placebo.
- ARIA with brain bleeding was seen in 17.3% of trial participants, compared with 9% of placebo group.
- Fortunately, most ARIA is asymptomatic, with only 2.8% of patients experiencing symptoms such as headache, confusion or visual disturbance.
Lecanemab label warnings include brain swelling and brain bleeding. People who are apolipoprotein E ε4 allele carriers are at an increased risk of Alzheimer’s disease and are at greater risk of ARIA while being treated with lecanemab. Recommended us guidelines also caution against taking blood thinners while on the medication.
Refer a patient
To refer a patient to Norton Neuroscience Institute Memory Center, visit Norton EpicLink and open an order for Adult Neurology.
ARIA
According to the American Journal of Roentgenology, ARIA describes MRI findings observed in patients receiving investigational anti–amyloid beta (Aβ) immunotherapies for Alzheimer’s disease. ARIA falls broadly into two categories: ARIA with edema and effusion (ARIA-E) or ARIA with microhemorrhages and superficial siderosis (ARIA-H). The condition is often asymptomatic and self-resolving, although it can become serious.
Access to lecanemab and next steps
Lecanemab is indicated for those with very early or mild Alzheimer’s disease. An assessment of medical history, MRI and minor blood work are required to determine whether a patient is eligible. Additional testing to determine the presence of pathologic amyloid is necessary, and most often accomplished through a spinal fluid examination, through a positron-emission tomography (PET) scan may become more widely used in the future. Finally, a genetic test (ApoE genotyping) can determine the risk of amyloid-related abnormalities (ARIA).
Donanemab, a second anti-amyloid agent, could receive FDA approval later in 2024. This medication will have the advantage of requiring infusions every four weeks, but comes with a higher risk of ARIA.
“We currently treat around 70 patients, and we add two or three patients per week,” Dr. Cooper said. “Our experience has been largely in line with expectations from the trials.”

